
Facts About the Periodic Table for Kids
Posted by Admin / in Science Facts
The periodic table represents all of the elements that scientists and chemists have discovered. These elements are are unique and, currently scientists think that it is not possible to break these elements down into any smaller units. Find out some facts about the history of the periodic table and how the elements are organized on the periodic table.
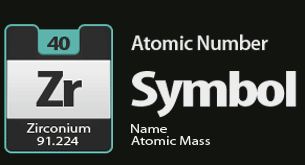
The Periodic Table of Elements lists the Chemical Symbol, the Atomic Number and the Atomic Mass of each Element
Periodic Table of Elements Facts
- Elements are the building blocks of all mater.
- The periodic table of elements contains 109 different elements.
- Each element contains a unique type of element. Since each element is different, we know there are 109 unique types of atoms.
- 90 of the 109 elements are found in nature.
- 19 of the 109 elements are only created in a laboratory.
- The one or two letter symbol listed for each element on the periodic table is called the chemical symbol. For example, the element chlorine is listed as Cl.
- Early scientists broke down the number of elements in our world to 4 elements including fire, earth, air and water.
- During the Bronze Age copper, arsenic and gold gained widespread use and were likely isolated as elements because of their value.
- Scientists in the 17th century began to break the number of elements down further.
- In 1766 Henry Cavendish discovered the air (atmosphere) contained more than one element. This was the first discovery that there were separate gas elements
- By the start of the nineteenth century, more than 50 elements were known by scientists.
- In 1869 Dmitri Mendeleev published the first periodic table of elements. Mendeleev's periodic table contained 63 known elements.
- The first periodic table also included blanks for unknown elements because Mendeleev understood that there were repeating patterns of elements.
- Although it has been expanded, the original concept and organization of Mendeleev's periodic table of elements is still used today.
-
The Periodic Table of Elements is grouped by Metals, Non-Metals, Semimetals and Noble Gases
-
Metals - These elements conduct heat and electricity well. Shaping this element into sheets or wire is possible.
-
Non-Metals - These elements do not conduct heat and electricity. These elements are also not easily shaped.
-
Semimetals - Semimetals have properties of both metals and non-metals.
-
Nobel Gases - Noble Gases do not combine with other elements.
